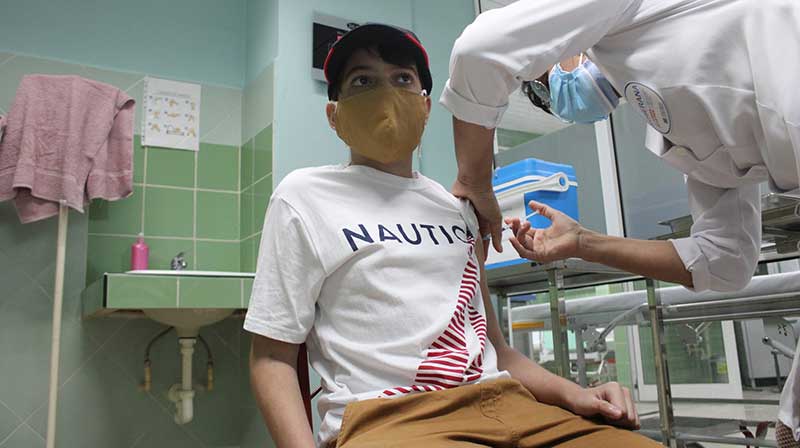
Havana, July 19 (ACN) The Cuban Finlay Vaccine Institute highlighted the high safety level proven by clinical trials with the COVID-19 candidate vaccine Soberana 02 in Cuban children.
On its Twitter account, the institution said that a total of 350 children are part of the study which has already administered a first dose of the locally developed candidate vaccine.
Cuba’s Regulatory Authority, the State Center for the Control of Medications, Equipment and Medical Appliances, authorized the clinical trials in children on June 10.
The study, which is applied in three doses, one every 28 days, is an urgent need given the increasing number of COVID -19 pediatric cases being registered on the island.
Sidebar

 Agencia Cubana de Noticias
Líder en información nacional
Agencia Cubana de Noticias
Líder en información nacional








Nos reservamos el derecho de no publicar los comentario que incumplan con las normas de este sitio