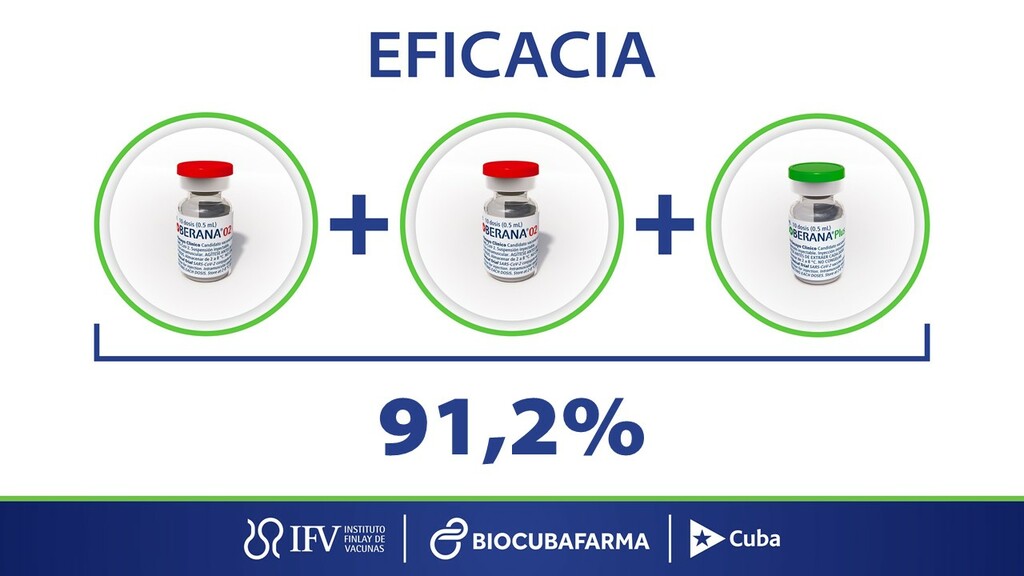
HAVANA, Cuba, Jul 8 (ACN) The general director of the Finlay Vaccine Institute, Vicente Vérez Bencomo, announced today that Soberana 02 in its three-dose scheme with Soberana Plus booster reached an efficacy of 91.2 percent (%) against the symptomatic disease, a figure higher than the one established by the World Health Organization (WHO) to consider anti-SARS-CoV-2 immunogens as effective vaccines.
The result was announced before Miguel Díaz-Canel Bermúdez, First Secretary of the Central Committee of the Communist Party of Cuba and President of the Republic, during the meeting of the Temporary Working Group to confront COVID-19, where the President congratulated the scientists and pointed out that this demonstrates the commitment in their work in these difficult circumstances.
Vérez Bencomo said that this number is really very high and is the reflection of a non-stop work during 59 weeks since they were summoned to achieve sovereignty with the vaccines.
He also said that the efficacy of the three-dose schedule was determined in the presence of the Beta variant of SARS-CoV-2, notified for the first time in South Africa, one of the most worrying ones recognized by the WHO, due to its high transmissibility.
This is a first step and much remains to be done to contain the transmission of the disease, we cannot rest until all Cubans are vaccinated and sure that they will not die in case they get sick, he said.
Last June 19, the IFV reported that in Phase III clinical trials the Soberana 02 vaccine candidate in its two-dose schedule showed 62 percent efficacy, in addition to a confidence interval higher than 40 percent, also above the WHO requirement of 30 percent.
Vérez Bencomo recently underlined the promise of implementing a heterologous vaccination scheme (with two vaccines), an innovative alternative used by Cuba since January, when this possibility had not yet been mentioned in the world.
Soberana 02 is the only conjugated anti-SARS-Cov-2 immunogen in the world, in which the virus antigen, the receptor binding domain (RBD), is chemically linked to the tetanus toxoid, while Soberana Plus includes two RBDs linked together with aluminum hydroxide and is the only vaccine project being studied in the convalescent population.
The latter formulation has proven to be a valuable immunity booster, both for people naturally vaccinated with the virus and for those who received other vaccine candidates, as is the case with Soberana 02.
IFV specialists have stated that it is more advisable to use Soberana Plus, which is less complex but very immunogenic, as it includes only the protein needed by the immune system to reactivate its memory cells.
Based on these results, the Center for State Control of Medicines, Equipment and Medical Devices will be able to officially register Soberana 02 plus Plus as a vaccine, issue the authorization for emergency use and thus move towards a future mass vaccination of the Cuban population.
Soberana 02 received, last June 29, the authorization for emergency use in the Islamic Republic of Iran, granted to the Pasteur Institute of Iran, which will commercialize the vaccine in that Iranian territory under the name Pasteur, within the framework of a collaboration agreement signed with the IFV.
In October 2020, Phase I trials with the vaccine candidate began, in December it moved to Phase II and on March 3 of this year it received authorization to advance to Phase III.
Its three-dose schedule is also currently being tested in children and adolescents through the Soberana-Pediatrics clinical trial.
Sidebar

 Agencia Cubana de Noticias
Líder en información nacional
Agencia Cubana de Noticias
Líder en información nacional








Nos reservamos el derecho de no publicar los comentario que incumplan con las normas de este sitio