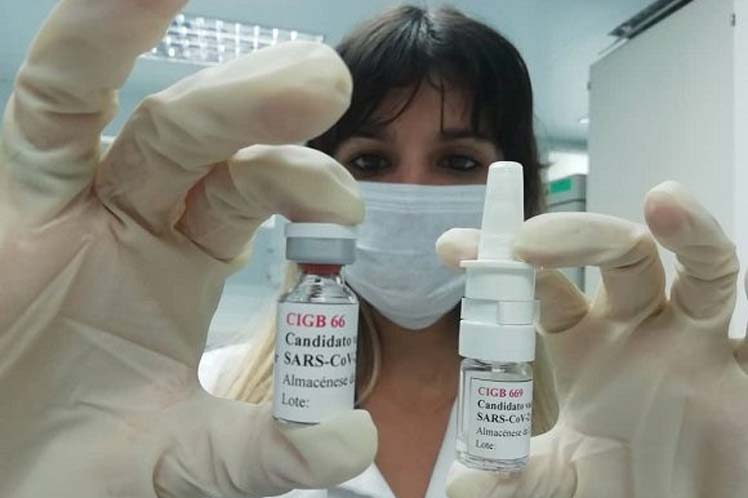
HAVANA, Cuba, Jan 18 (ACN) The clinical trials of the two vaccine candidates developed in the Center for Genetic Engineering and Biotechnology (CIGB) of Cuba concluded the short cycle of its phase I.
It was informed today to Prensa Latina by the leaders of the studies carried out for both candidates: Mambisa, mainly applied through the nasal way and Abdala through the intramuscular way.
The leader of the trial, Miladys Limonta, expressed that the first one has already finished the short cycle of the study, established for day zero, 14 and 28.
This candidate uses as antigen the AgsHB protein of the hepatitis B virus.
With a sample of 88 volunteers, the first immunizations conducted with Mambisa started on December 7 at Carlos J. Finlay Hospital in Havana.
The second candidate developed by the CIGB, Abdala, also finished the short cycle, in Saturnino Lora Hospital, in Santiago de Cuba province( east of the country).
The 132 volunteers who are part of this study have received, by intramuscular via, two doses of the vaccine candidate and placebo.
The beginning of clinical trials for Mambisa and Abdala was approved last November 27 by the Cuban Regulatory Authority of Medicines, Equipment and Medical Devices.
Cuba is also developing studies for other two candidates, Sovereign 01 and Sovereign 02, from the Finlay Institute of Vaccines.










Nos reservamos el derecho de no publicar los comentario que incumplan con las normas de este sitio