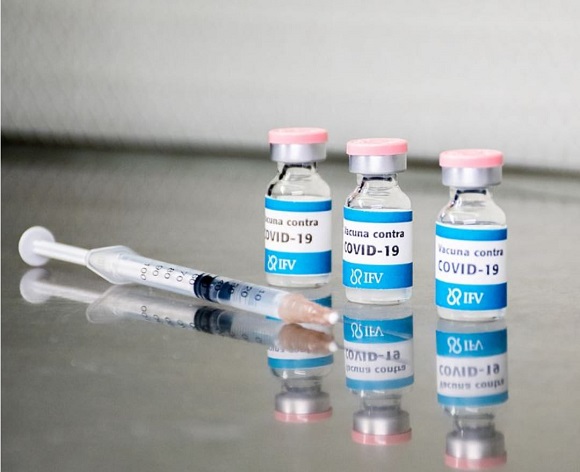
HAVANA, Cuba, Jan 18 (ACN) The vaccine candidate Soberana 02, developed by the Finlay Institute of Vaccines, started today the second stage of phase two of the clinical trials in the municipality of Plaza de la Revolución in Havana.
Some 405 volunteers will receive the first dose at the Polyclinic 19 de Abril, while more than 100 have already been vaccinated at the Clinica Uno in La Lisa, west of Havana.
Last November 2, the first stage of Soberana 02's phase one started with the participation of 40 volunteers and the preliminary results of the study demonstrated the effectiveness of the Cuban medication.
Once this phase two is completed, it is expected that the candidate will move on to a phase three including about 150,000 people.
On the other hand, today Prensa Latina reported that the clinical trials of the two vaccine candidates developed at the Center for Genetic Engineering and Biotechnology (CIGB), in Havana concluded the short cycle of its phase one, in reference to Mambisa, applied mainly through the nose, and Abdala, through the intramuscular way.
Sidebar

 Agencia Cubana de Noticias
Líder en información nacional
Agencia Cubana de Noticias
Líder en información nacional








Nos reservamos el derecho de no publicar los comentario que incumplan con las normas de este sitio