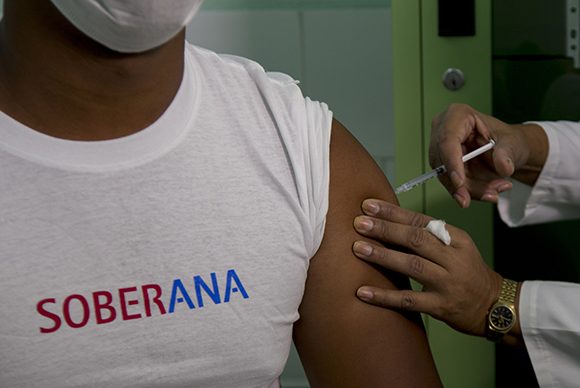
HAVANA, Cuba, Jan 9 (ACN) The Finlay Institute of Vaccines, belonging to the Group of Biotechnological and Pharmaceutical Industries of Cuba, BioCubaFarma, signed a bilateral agreement with Iran to complement clinical evidence of the Soberana 02 vaccine candidate.
Biocubafarma stated that this cooperation will allow a faster progress in the immunization against COVID-19 in both countries.
BioCubaFarma in a message published in Twitter highlights that the Pasteur Institute of Iran has a long history of collaboration with institutions of the Group and today the friendship ties between the two nations are strengthened.
The Center of Molecular Immunology (CIM) commented that the complementation of scientific-technological capacities is vital to promote the accelerated development of effective therapies.
In 2020, four vaccination candidates against COVID-19 were approved in Cuba, which are in several phases of clinical trials.
Last December 17, Soberana 02 passed to phase II of clinical trials, for which it constitutes the first Latin American drug to advance to that stage.
The vaccine candidate, in that phase, began to be applied in the last weeks of 2020 by means of vaccination, a process that passed with normality and with the strict fulfillment of the norms and sanitary protocols established for such cases.
Soberana 02 is a conjugated vaccine, in which the virus antigen, the receptor binding domain (RBD), is chemically linked to the tetanus toxoid.
Besides, it is innovative, as it has no precedents among all vaccines being developed around the world to fight the new coronavirus
Sidebar

 Agencia Cubana de Noticias
Líder en información nacional
Agencia Cubana de Noticias
Líder en información nacional








Nos reservamos el derecho de no publicar los comentario que incumplan con las normas de este sitio